The Need for Rust Inhibitors for Vehicles in Sub Zero Temperatures
Your car — an investment second only to your home (unless you’ve paid college tuition) — is rotting out from under you.
Road brine is a treatment for the highways and local roads of the nation, especially those that experience sub-zero temperatures in the winter. It stops us from slipping and sliding when out and about taking our children to school or commuting to work. But there is a hidden cost of using road brine. One that brings the need for rust inhibitors for cars and trucks or oil-based rustproofing to the fore. And the problem is literally eating away at the underside of many cars, trucks and other vehicles across the USA – and the rest of the world at the underside of many cars, trucks and other vehicles across the USA – and the rest of the world.
Road brine looks like quite a natural solution to keeping the roads safe. It used salt and water – the combination known as brine – to stop the road from freezing and provide conditions that are safe to drive in. You may spend time looking after your car on the inside and the bodywork all year around. You will even get that winter check before the cold really starts to kick in to make sure you are on top of the maintenance of your vehicle. But I bet you have never considered oil-based rustproofing with rust inhibitors for cars and trucks to solve a problem.
Road brine eats away at the underside of your vehicle. Car rust and corrosion are caused by acid created when a salt is dissolved by the moisture in the air. Rock salt remains a crystal until the humidity reaches 70 percent, which doesn’t happen much during the winter. But magnesium chloride dissolves when there is only about 20 to 30 percent humidity. “Which means that your vehicle if magnesium chloride is sprayed on it, is wet constantly,” Baboian said. The acid stays on your car and slowly eats away at the paint and metal.
The salt accelerates the oxidation on the metal chassis and starts a rusting process that accelerates every time you take the car out in these conditions. Let’s face it – for many of us, there is no choice but to use our vehicle day after day. When we do, the salt sits there, unwashed, and corrodes. The risk is worsened performance and a safety problem that you can’t see unfolding. There is a huge worry in this, as corrosion from road brine is linked to tens of thousands of accidents across the United States. Examples can be found all over.
The other issue with road brine is the fact that magnesium is added to the mix, in small quantities. Although there isn’t much, it stops the vehicle from drying out, leaving the acidity from the salt to do a lot of harm to your car. You should look at how you can address this to prolong the life of your vehicle and to stay safe on the road.
If you are a business owner, then you want your employees and all in the vehicle to stay safe.
If you are a car owner, then you use your vehicle to transport all that is important to you.
Either way, there is a huge incentive to look into oil-based rustproofing with rust inhibitors for cars and trucks or oil-based rustproofing as a solution to this problem. Let’s take a look at what these items mean in reality.
Rust inhibitors for cars and trucks – what are they all about?
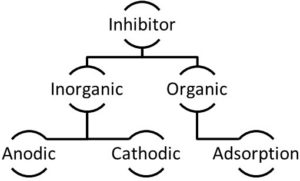
The corrosion inhibitors can be chemicals either synthetic or natural and could be classified
Figure 1. Classification of inhibitors
by:
- the chemical nature as organic or inorganic;
- the mechanism of action as anodic, cathodic or an anodic-cathodic mix and by adsorption
action, or; as oxidants or not oxidants.
In general, inorganic inhibitors have cathodic actions or anodic. The organics inhibitors
have both actions, cathodic and anodic, and the protective by film adsorption.
This chapter is subdivided in according to the classification of the inhibitors shown on the
Rust inhibiting coating for cars or trucks are chemical compounds that are added to other liquids in a vehicle to aid the protection against rust. They can work from the inside or outside to stop rust at the source. There are some that can be added to gas or oil to stop oxidization inside the engine. But that is a tiny part of the problem and these don’t look at the real issue.
The underside of a vehicle is where the corrosion happens and causes the concerning damage. So, the majority of rust inhibitors for cars and trucks are sprays that can be applied on a regular basis. There are scores of them available here and these can be bought at a relatively small cost. The recommendation is that these are carried out annually at least, with the Spring months being the ideal point in time to apply this spray. This leaves the car protected in some way, so think about how you can make all of this happen.
Fortunately, this approach can be accomplished by a do-it-your-self here is a How-to video
It is a little messy operation. Applying oil-based rustproofing with rust inhibitors for cars and trucks means getting your car raised in the air then applying the spray to the underside of the vehicle. The mechanics of this means you will need a jack or other device and you will be under the vehicle spraying. This isn’t for everyone So you can find an oil-based rustproofing professional to apply it for you here
It isn’t 100% . You cannot beat mother nature but you can slow her down. The range of sprays on the market suggests that the problem is big. The protection they offer really depends on how well you apply it. If you are concerned about road brine and the damage it can cause (and you should be) then you want a solution that resolves the problem. Applying oil-based rustproofing with rust inhibitors for cars and trucks can help extend the life of your second most expensive asset.
NH oil-based rustproofing with rust inhibitors These sprays can offer varying degrees of protection. If you take a look at the reviews for our products, You will find we have made substantial progress in extending the life of vehicles and protecting them from Road brine
What can you do? Oil-Based Rustproofing and how it can help?
One of the major advantages of oil-based rustproofing with rust inhibitors for cars and trucks is the fact that you are creating a barrier from moisture and Road brine. NH oil-based rustproofing with rust inhibitors will displace moisture and cut off oxygen. Take either out of the equation and oxidation cannot occur. NH Oil Undercoating will creep and penetrate deep into the body cavities, spot welds, and seems. Those areas up prone to hold moisture, unprotected, and generally where rust begins.
NH Oil Vehicle Undercoating® has corrosion inhibitors that will remain present throughout the year. Look for oil-based rustproofing to deliver you an effective solution that can extend the life of the vehicle.
If you don’t want to trap moisture, then you will need to steer clear of rubberized undercoating for cars and trucks and look for oil-based rustproofing instead. This is an application of an oil-based rustproofing product that coats the underside of the vehicle – protecting all of that metal from the effects of corrosion from road brine. Unlike the other solutions, NHOU oil-based rustproofing will protect you fully and leave your vehicle safe from the potential threat of corrosion.
Road brine is a problem that is only just rearing its head in the consciousness of the general public. But it is a massive potential problem that can change the way your car or truck performs or provides safety. Don’t take a risk with the safety of your vehicle when it is on the road. There is far too much at stake. Look at oil-based rustproofing to deal with this issue and put you in the best place possible.
When it comes to auto corrosion prevention… We’ve got you covered!
See the difference for yourself!
All our specialized products will protect your vehicle against auto corrosion damage caused by salt, liquid de-icing, and winter driving conditions. They are designed to penetrate the seams and crevices. It eliminates moisture. It actually stops rust and corrosion in all metals, (when present) it protects vinyl and plastic surfaces from UV rays and improves the function of moving parts through lubrication.
The fact is, a new vehicle is subjected to thousands of spot welds and numerous bends and folds during production.
This process damages the pre-coated metal, allowing exposure to the corrosion process. Besides cosmetic damage, corrosion also weakens a vehicle’s structural integrity and can affect steering and suspension components.
NH Oil Vehicle Undercoating® Services & products, undercoating for cars & trucks will perform because we use the products that we create, we have a unique opportunity to not only see the performance but to design the products to be user-friendly.
The only product on the market formulated by the people who apply it; from us to you!
Corrosion-Rust inhibitors for cars and truck in-depth;
Corrosion rust Inhibitors – Principles, Mechanisms and
Applications
Camila G. Dariva and Alexandre F. Galio
Additional information is available at the end of the chapter http://dx.doi.org/10.5772/57255
1. Introduction
Corrosion processes are responsible for numerous losses mainly in the industrial scope. It is clear that the best way to combat it is prevention.
Among the various methods to avoid or prevent destruction or degradation of the metal surface, the corrosion inhibitor is one of the best known methods of corrosion protection and one of the most useful on the industry. This method is following stand up due to low cost and practice method. [1] [2] [3] [4]
Important researches have been conducted with government investment mainly in large areas such as the development construction of new pipelines for shale gas and growth in construction. The focus of this researches has to be the inhibitors’ applications in water and concrete for the protection of metals. [5]
Historically, inhibitors had great acceptance in the industries due to excellent anti-corrosive proprieties. However, many showed up as a secondary effect, damage the environment. Thus the scientific community began searching for friendly environmental inhibitors, like organic inhibitors. [6] [7] [8] [9] [10] [11] [12] [13]
This chapter presents a revision of the corrosion inhibitor’s applications mainly the novel compositions environmentally friendly. Is describes the mechanisms of action of inhibitors, main characteristics, environmental impact, technical analysis, and calculation of efficiency.
1.1. Mechanisms of actions of Rust inhibitors
Inhibitors are substances or mixtures that in low concentration and in an aggressive environment inhibit, prevent or minimize corrosion. [2]
© 2014 Dariva and Galio; licensee InTech. This is a paper distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
Generally, the mechanism of the inhibitor is one or more of three that are cited below:
- the inhibitor is chemically adsorbed (chemisorption) on the surface of the metal and forms a protective thin film with inhibitor effect or by a combination between inhibitor ions and metallic surface;
- the inhibitor leads a formation of a film by oxide protection of the base metal;
- the inhibitor reacts with a potential corrosive component present in aqueous media and the product is complex. [4] [14] [15]
1.2. Historic review of rust inhibitors
There are many industrial systems and commercial applications that inhibitors are applicable, such as cooling systems, refinery units, pipelines, chemicals, oil and gas production units, boilers and water processing, paints, pigments, lubricants, etc. [16]
There is evidence of the use of inhibitors since the early XIX century. At that time they were already used to protect metals in processes such as acid picking, protection against aggressive water, acidified oil wells, and cooling systems. Since the years 1950’s and 1960’s, there were significant advances in the development of technology for corrosion inhibitors as the application of electrochemistry to evaluate corrosion inhibitors. [17]
Recent studies estimate that the U.S. demand for corrosion inhibitors will rise 4.1% per year to USD$ 2.5 billion in 2017. In 2012 they estimated that the market demand of inhibitors was divided on 26.6% to refining petroleum, 16.9% utilities, 16.7% gas and oil production, 15.3% chemical, 9.5% metals, 7.1% pulp and paper, and 8.0% other. [5]
Nowadays, due to changes occurred in the market of corrosion inhibitors, some industrial corrosion inhibitors are being unused. Due to the high toxicity of chromate, phosphate and arsenic compounds, related to various environmental and health problems, strict international laws were imposed. Reducing the use of these and therefore increasing the need for the development of other inhibitors to supply the lack in this area. Should, however, present similar anti-corrosive properties similar to a chromate inhibitor. [18]
An important number of papers have been published with the intention of developing environmentally-friendly corrosion inhibitors and a lot of research has been doing to development of the called “green” corrosion inhibitors.[19] Also, has been increasing research in natural products, such as plant extracts, essential oils, and purified compounds to obtain environmentally-friendly corrosion inhibitors. [20]
The first evidence of natural product use as corrosion inhibitors is the 1930’s. When extracts of Chelidonium majus (Celadine) and other plants were used for the first time in H2SO4 pickling baths. Successful developments of researches to obtain natural corrosion inhibitors are growing as quickly as the environmental consciousness is gaining ground.[21]
Chromates as active inhibitors are being replaced by other components such as molybdate compounds and rare earth metal salt, like cerium chloride. Also, drugs have been studied as corrosion inhibitors. [22] [23] [24]
2. Rust Inhibitors classifications
classified by:
- the chemical nature as organic or inorganic;
- the mechanism of action as anodic, cathodic, or an anodic-cathodic mix and by adsorption action, or;
- as oxidants or not oxidants. [4]
In general, inorganic inhibitors have cathodic actions or anodic. The organics inhibitors have both actions, cathodic and anodic and the protective by film adsorption.
This chapter is subdivided in according to the classification of the inhibitors shown on Figure 1 The corrosion inhibitors can be chemicals either synthetic or natural and could be
Figure 1. Classification of inhibitors
2.1. Inorganic rust inhibitors
2.1.1. Anodic rust inhibitors
Anodic inhibitors (also called passivation inhibitors) act by reducing the anodic reaction, that is, blocks the anode reaction and supports the natural reaction of the passivation metal surface, also, due to the forming a film adsorbed on the metal. In general, the inhibitors react with the corrosion product, initially formed, resulting in a cohesive and insoluble film on the metal surface. [4] [24]
Figure 2 shows a potentiostat polarization diagram of a solution with behavior inhibitor anodic. The anodic reaction is affected by the corrosion inhibitors and the corrosion potential of the metal is shifted to more positive values. As well, the value of the current in the curve decreases with the presence of the corrosion inhibitor.
Figure 2. Potentiostatic polarization diagram: electrochemical behavior of a metal in a solution with anodic inhibitor (a) versus without inhibitor (b).
The anodic inhibitors react with metallic ions Men+ produced on the anode, forming generally, insoluble hydroxides which are deposited on the metal surface as insoluble film and impermeable to metallic ion. The hydrolysis of inhibitors results in OH– ions. [4] Figure 3 shows how is the mechanism of the anodic inhibitory effect.
Figure 3. Illustration of anodic inorganic inhibitors’ effect and their mechanism of action.
When the concentrations of inhibitor become high enough, the cathodic current density at the primary passivation potential becomes higher than the critical anodic current density, that is, shift the potential for a noble sense, and, consequently, the metal is passivated. [25] [26]
For the effect of the anodic inhibitors, it is very important that the inhibitor concentrations should be high enough in the solution. The inappropriate amount of the inhibitors affects the formation of film protection because it will not cover the metal completely, leaving sites of the metal exposed, thus causing localized corrosion. [4] [25] [26]
Concentrations below to the critical value are worse than without inhibitors at all. In general can cause pitting, due to reduction at the anodic area relative to cathodic, or can accelerate corrosion, like generalized corrosion, due to the full breakdown of the passivity. [25]
Some examples of anodic inorganic inhibitors are nitrates, molybdates, sodium chromates, phosphates, hydroxides and silicates.
2.1.2. Cathodic rust inhibitors
During the corrosion process, the cathodic corrosion inhibitors prevent the occurrence of the cathodic reaction of the metal. These inhibitors have metal ions able to produce a cathodic reaction due to alkalinity, thus producing insoluble compounds that precipitate selectively on cathodic sites. Deposit over the metal a compact and adherent film, restricting the diffusion of reducible species in these areas. Thus, increasing the impedance of the surface and the diffusion restriction of the reducible species, that is, the oxygen diffusion and electrons conductive in these areas. These inhibitors cause high cathodic inhibition. [4] [24] [27]
Figure 4 shows an example of a polarization curve of the metal on the solution with a cathodic inhibitor. When the cathodic reaction is affected the corrosion potential is shifted to more negative values.
Figure 4. Potentiostatic polarization diagram: electrochemical behavior of the metal in a cathodic inhibitors solution (a), as compared to the same solution, without inhibitor (b).
The cathodic inhibitors form a barrier of insoluble precipitates over the metal, covering it. Thus, it restricts the metal contact with the environment, even if it is completely immersed, preventing the occurrence of the corrosion reaction. Due to this, the cathodic inhibitor is independent of concentration, thus, they are considerably more secure than an anodic inhibitor. Figure 5 shows the illustration of the mechanical effect of cathodic inhibitors to restrain the corrosion process. [4]
Figure 5. Illustration has shown the mechanism of actuation of the cathodic inhibitors.
Some examples of inorganic cathodic inhibitors are the ions of the magnesium, zinc, and nickel that reacts with the hydroxyl (OH–) of the water forming the insoluble hydroxides as (Mg(OH)2, Zn(OH)2, Ni(OH)2) which are deposited on the cathodic side of the metal surface, protecting it. [4] Also can be cited polyphosphates, phosphonates, tannins, lignins [15] and calcium salts as examples that present the same reaction mechanism.
It seen in hard waters a kind of this mechanism of inhibiting, due to the effect of the magnesium or calcium bicarbonate on it. When temporary hard water flows over the metal it can assist on the nucleation of carbonates, allowing the reactions near to the equilibrium and forming precipitations on the metal surface. These precipitations, like a CaCO3, cover the cathodic area, protecting the metal. So these cathodic inhibitor depends only on the chemistry of the water, is not due to the metal composition, because of this they are applicable to all metals. [4] [25]
As an example, it may be mentioned the oxides and salts of antimony, arsenic, and bismuth, which are deposited on the cathode region in acid solutions. These cathodic inhibitors minimize the release of hydrogen ions due to phenomena that can difficult the discharge of the hydrogen, called overvoltage.
2.2. Organic rust inhibitor
Organic compounds used as inhibitors, occasionally, act as cathodic, anodic or together, as cathodic and anodic inhibitors, nevertheless, as a general rule, act through a process of surface adsorption, designated as a film-forming. Naturally, the occurrence of molecules exhibiting a strong affinity for metal surface compounds showing good inhibition efficiency and low environmental risk. [28] These inhibitors build up a protective hydrophobic film adsorbed molecules on the metal surface, which provides a barrier to the dissolution of the metal in the electrolyte. They must be soluble or dispersible in the medium surrounding the metal. [4]
In Figure 6, which shows a theoric potentiostatic polarization curve, it can be seen that the effect of the solution containing organic inhibitor on the metal presents an anodic and cathodic behavior. After the addition of the inhibitor, the corrosion potential remains the same, but the current decreases from Icor to I’cor.
Figure 6. Theoretical potentiostatic polarization diagram: electrochemical behavior of a metal on a solution containing a cathodic and anodic inhibitor (a) compared to the same solution without the inhibitor (b).
Is shown in Figure 7 the mechanism of actuation of organic inhibitors, when it is adsorbed to the metal surface and forms a protector film on it.
Figure 7. Illustration of the mechanism of actuation of the organic inhibitor: acting through adsorption of the inhibitor on the metal surface. Where the Inh represents the inhibitor molecules.
The efficiency of an organic inhibitor depends of the:
- chemical structure, like the size of the organic molecule;
- aromaticity and/or conjugated bonding, as the carbon chain length;
- type and number of bonding atoms or groups in the molecule (either π or σ);
- nature and the charges of the metal surface of adsorption mode like bonding strength to the metal substrate;
- the ability for a layer to become compact or cross-linked,
- the capability to form a complex with the atom as a solid within the metal lattice;
- type of electrolyte solution like adequate solubility in the environment. [16]
The efficiency of these organic corrosion inhibitors is related to the presence of polar functional groups with S, O or N atoms in the molecule, heterocyclic compounds and pi electrons, generally have hydrophilic or hydrophobic parts ionizable. The polar function is usually regarded as the reaction center for the establishment of the adsorption process.[4] [28]
The organic acid inhibitor that contains oxygen, nitrogen and/or sulfur is adsorbed on the metallic surface blocking the active corrosion sites. Although the most effective and efficient organic inhibitors are compounds that have π-bonds, it presents biological toxicity and environmental harmful characteristics. [29]
Due to the metal surface covered is proportional to the inhibitor concentrates, the concentrations of the inhibitor in the medium is critical. [24] [30]
Some examples are amines, urea, Mercaptobenzothiazole (MBT), benzotriazole e toliotriazol, aldehydes, heterocyclic nitrogen compounds, sulfur-containing compounds and acetylenic compounds and also ascorbic acid, succinic acid, tryptamine, caffeine and extracts of natural substances. [4] [25] [28]
There are still some inhibitors that act in the vapor phase (volatile corrosion inhibitor). Some examples are: dicicloexilamônio benzoate, diisopropyl ammonium nitrite or benzoate, ethanolamine benzoate or carbonate and also the combination of urea and sodium nitrite. [4] [24]
3. Techniques for analysis of rust inhibitors
The most useful technique to analyze the effectiveness of an inhibitor is weight loss experiment and electrochemical measurements, like the polarization curve method and the impedance measurement analysis. In addition, microscopy techniques are used to characterize the corrosion process.
4. Considerations to employ rust inhibitors
For all types of inhibitors, we should consider some environmental action factors because of some elements such as metals, pH, composition, impurities, agitation, temperature, the geometry of the system, the concentration of inhibitor and the mixture of one or more inhibitors may change the anti-corrosive mechanism. [4] [23] [31] [33]
To the employment of the inhibitors is quite satisfying that certain factors should be seen as the real cause of the corrosion, the cost X benefit and possible interactions of the inhibitor with the environment, such as the influence of a catalyst, deposition or contamination. Four fundamental aspects must be analyzed to obtain a satisfactory result from the use of the inhibitor.
5. Rust inhibitor efficiency
The inhibitor efficiency could be measured by the following equation:
Ri – Ro ´100 (1)
E =f
Ro
where Ef is inhibitor efficiency (percentage), Ri is corrosion rate of metal with inhibitor and Ro is corrosion rate of metal without inhibitor.[38]
6. Industrial application
Acid pickling: Prevent the attack in the metal due to the acid solution in which metal gets cleaned of mill scale (bark lamination), and also prevented the subsequent hydrogen evolution inhibitors are added, typically organic, must be soluble or dispersed in the solution. Examples: thiourea and amino and its derivatives, propargyl alcohol. [4]
Oil industry: sodium carbonates or organic amines complex is employed to reduce the corrosive effect of CO2, H2S and organic acids, enabling the use of cheaper materials and less resistance to corrosion in wells extracting crude oil. Pipes for gasoline and kerosene are employed sulfonated oils, sodium nitrite. Oil well uses up fatty amines, fatty acids, imidazolines, and quaternary ammonium salts. Internal pipe corrosion occurs in wet gas transportation due to condensation of water containing dissolved corrosive gases. Corrosion is caused by the dissolution of corrosive gases, such as carbon dioxide and hydrogen sulfide as well as condensation of acid vapors [42].
Water transmission and distribution systems: is used corrosion inhibitor in combination with pH adjusters and alkalinity control towards efficient protection [32] The most common inhibitors are phosphates, amines volatiles (cyclohexylamine, morphine) [4]
Concrete: To improve the durability of reinforced concrete structures, which are impaired due to the high alkalinity, are used corrosion inhibitors, mixed with cement or concrete paste. An example is phosphate ion. [33]
Boiler: Thermoelectric use, in general, Ammonia, Cyclohexylamine, alkanol, and Morpholine as inhibitors in boilers in various processes. The inhibitors, also, are added by the hydrochloric acid used for the solubilization of limescale to prevent the attack on pipes.
7. Conclusion
Inhibitors are a great method of preventing corrosion and are easy to apply. Has an application in a wide range of sectors
The knowledge of the method of the action facilitates the choice of the inhibitors, improves efficiency, avoids the process is impaired and side effects.
It is important in the choice of inhibitor, ascertain the subsequent effects of this on the environment.
The search for environmentally friendly inhibitors has shown excellent results, outperforming conventional inhibitors.
Reference organic inhibitors newly developed
| 7 | K2Cr2O7 | 10-4 M | 97.31 |
| 7 | Cerium
dibutylphosphate (Ce(dbp)3) |
10-4 M | 94.10 |
AA2024-T3 0,1 M NaCl[23]
| 7 | Cerium chloride (CeCl3) | 10-4 M | 73.90 | |||||||
| AA5754 | 3% NaCl | 6.3 | Laurus nobilis L. oil | 50ppm | 84.3 | [43] | ||||
| Al | 3% NaCl | 6.3 | Laurus nobilis L. oil | 50ppm | 89.9 | [43] | ||||
| AA5083 | 3.5% NaCl | 5.5 | CeCl3 | 500ppm | 297 | [44] | ||||
| LaCl3 | 500ppm | 113 | [44] | |||||||
| Steel | 1M HCl | – | N. cadamba Bark extract | 5mgL-1 | 91.0 | [45] | ||||
| Steel | 1 M HCl | – | VSBH (C31H47O4N2Br) | 400 ppm | 95.0 | [46] | ||||
| Steel | 88% phosphoric acid | – | Dried Zenthoxylum-alatum
plant fruits |
2400ppm | 98.0 | [47] | ||||
| Steel | 0.5 M H2SO4 | – | Polyacrylamide grafted Okra mucilage(O-g-PAM) | 100ppm | 94.4 | [48] | ||||
| Copper | 0.5M HCl | – | Chitosan | 8 × 10−6 | 92.0 | [29] | ||||
| Cu-10Al-5Ni alloy | 3.5% NaCl | – | Cysteine | 6mM/L | 96.0 | [49] | ||||
| N-acetylcysteine | 6mM/L | 87.6 | [49] | |||||||
| alloy | environmental | pH | inhibitor | concentration | efficiency | reference | ||||
| Methionine | 6mM/L | 76.7 | [49] | |||||||
| Cu | 0.6M NaCl | – | Cysteine | 16mM | 76.5 | [50] | ||||
| Cu | 1M HCl | – | Cysteine | 18mM | 84.13 | [50] | ||||
| AZ91D | 0.05 wt.% NaCl | 9 | Paeonol | 50ppm by wt | 90 | [51] | ||||
| AZ91D | ASTM D1384-87 | 8.2 | 8-hydroxyquinoline | Saturated with
8HQ |
82 | [52] | ||||
| AZ91D alloy | 0.05 wt.% NaCl | 6.8 | 5,10,15,20-
tetraphenylporphyrin (TPP) |
5ppm | 84 | [53] | ||||
Table 1. Inhibitors organics for aluminum, steel, copper, magnesium and its alloys at room temperature.
Author Details
Camila G. Dariva and Alexandre F. Galio*
*Address all correspondence to: afgalio@ufrgs.br
PPEng – CAPES and Universidade Federal do Pampa Bagé/RS, Brazil
References
- S. Al-Otaibi, A. M. Al-Mayouf,. M. Khan, A. A. Mousa, S. A. Al-Mazroa e H. Z. Alkhathlan, “Corrosion inhibitory action of some plant extracts on the corrosion of mild steel in acidic media,” Arabian Journal of Chemistry, pp. 1-7, 2012.
- B. Obot, N.O. Obi-Egbedi, S.A. Umoren, “Antifungal drugs as corrosion inhibitors for aluminum in 0.1 M HCl,” Corrosion Science, vol. 51, issue 8, pp. 1868-1875, 2009.
- Yıldırım, M. Çetin, “Synthesis and evaluation of new long alkyl side chain acetamide, isoxazolidine and isoxazoline derivatives as corrosion inhibitors,” Corrosion Science, vol. 50, issue 1, pp.155-165, 2008.
- Gentil, Corrosão, 4ª ed., Rio de Janeiro: LTC, 2003.
- Finishing, “pfonline,” Finishing Industry, 03 06 2013. [Online]. Available: http:// www.pfonline.com/news/us-demand-for-corrosion-inhibitors-to-reach-25-billionin-2017. [Access on 10 07 2013].
- Negm, Nabel A.; Kandile, Nadia G.; Badr, Emad A.; Mohammed, Mohammed A. Gravimetric and electrochemical evaluation of environmentally friendly nonionic corrosion inhibitors for carbon steel in 1 M HCl. Corrosion Science, vol. 65, pp. 94-103, 2012.
- M. Abdel-Gaber, B.A. Abd-El-Nabey, E. Khamis, D.E. Abd-El-Khalek. A natural extract as scale and corrosion inhibitor for steel surface in brine solution. Desalination. vol. 278, Issue 1-2, pp. 337-342, 2011.
- Salasi, M.; Sharabi, T.; Roayaei, E.; Aliofkhazraei, M. “The electrochemical behavior of environment-friendly inhibitors of silicate and phosphonate in corrosion control of carbon steel in soft water media,” Materials Chemistry and Physics, vol. 104, Issue 1, pp. 183-190, 2007.
- Blustein, R. Romagnoli, J.A. J. “Zinc basic benzoate as eco-friendly steel corrosion inhibitor pigment for anticorrosive epoxy-coatings,” Colloids and Surfaces A: Physicochemical and Engineering Aspects, vol. 290, Issue 1-3, pp. 7-18, 2006.
- Bommersbach, C. Alemany-Dumont, J.P. Millet, B. Normand. “Formation and behavior study of an environment-friendly corrosion inhibitor by electrochemical methods,” Electrochimica Acta, vol. 51, Issue 6, pp. 1076- 1084, 2005.
- Lecante, F. Robert, P.A. Blandinières, C. Roos. “Anti-corrosive properties of S.
tinctoria and G. ouregou alkaloid extracts on low carbon steel,” Current Applied Physics, vol. 11, Issue 3, pp. 714-724, 2011.
- Radojčić, K. Berković, S. Kovač, J. Vorkapić-Furač. “Natural honey and black radish juice as tin corrosion inhibitors,“ Corrosion Science, vol. 50, Issue 5, pp. 1498-1504, 2008.
- C. Okafor, M.E. Ikpi, I.E. Uwah, E.E. Ebenso, U.J. Ekpe, S.A. Umoren. “Inhibitory action of Phyllanthus amarus extracts on the corrosion of mild steel in acidic media,” Corrosion Science, vol. 50, Issue 8, pp. 2310-2317, 2008.
- Hong Ju, Zhen-Peng Kai, Yan Li, “Aminic nitrogen-bearing polydentate Schiff base compounds as corrosion inhibitors for iron in acidic media: A quantum chemical calculation,” Corrosion Science, vol. 50, Issue 3, pp. 865-871, 2008.
- V. Ramanathan, Corrosão e seu controle, São Paulo: Hemus, 1988.
- SANYAL, “Organic compounds as corrosion inhibitors in different environments A review,” Progress in Organic Coatings, vol. 9, pp. 165-236, 1981.
- Pourbaix. “Applications of electrochemistry in corrosion science and in practice,” Corrosion Science, vol. 14, pp. 25-82, 1974.
- S. Saji, “A review on recent patent in corrosion inhibitor,” Recent Patents on Corrosion Science, vol. 2, pp. 6-12, 2010.
- El Bribri, M. Tabyaoui, B. Tabyaoui, H. El Attari, F. Bentiss,“The use of Euphorbia falcata extract as eco-friendly corrosion inhibitor of carbon steel in hydrochloric acid solution,” Materials Chemistry and Physics, vol. 141, issue 1, pp. 240-247, 2013.
- Znini, L. Majidi, A. Bouyanzer, J. Paolini,. J. -M. Desjobert, J. Costa e B. Hammouti, “Essential oil of salvia aucheri mesatlantica as a green inhibitor for the corrosion of steel in 0.5M H2SO4,” Arabian Journal of Chemistry, vol. 5, n. 4, pp. 467-474, 2012.
- Pandian Bothi Raja, Mathur Gopalakrishnan Sethuraman,“Natural products as corrosion inhibitor for metals in corrosive media — A review,” Materials Letters, vol. 62, issue 1, pp. 113-116, 2008.
- L. Twite, G.P. Bierwagen,“Review of alternatives to chromate for corrosion protection of aluminum aerospace alloys,” Progress in Organic Coatings, vol. 33, issue 2, pp. 91-100, 1998.
- J. García, T.H. Muster, Ö. Özkanat, N. Sherman, A.E. Hughes, H. Terryn, J.H.W. de Wit, J.M.C. Mol, “The influence of pH on corrosion inhibitor selection for 2024-T3 aluminium alloy assessed by high-throughput multielectrode and potentiodynamic testing,” Electrochimica Acta, vol. 55, pp. 2457-2465, 2010.
- R. Roberge, Handbook of corrosion engineering, New York: Mc Graw Hill Handbook, 1999.
- C. Dutra e L. D. P. Nunes, Proteção catódica técnicas de combate a corrosão, 5 ed.,Rio de Janeiro: interciências, 2011.
- Bardal, Corrosion and protection, London: Springer, 2004.
- Talbot e J. Talbot, Corrosion science and technology, Florida: CRC Press, 2000.
- Aprael S. Yaro, Anees A. Khadom, Rafal K. Wael, “Apricot juice as green corrosion inhibitor of mild steel in phosphoric acid,” Alexandria Engineering Journal, vol 52, issue 1, pp. 129-135, 2013.
- Mahmoud N. El-Haddad, “Chitosan as a green inhibitor for copper corrosion in acidic medium,” International Journal of Biological Macromolecules, vol. 55, pp 142-149, 2013.
- El-Sayed M. Sherif, “Effects of 2-amino-5-(ethylthio)-1,3,4-thiadiazole on copper corrosion as a corrosion inhibitor in 3% NaCl solutions,” Applied Surface Science, vol 252, issue 24, pp. 8615-8623, 2006.
- Daobing Huang, Junying Hu, Guang-Ling Song, Xingpeng Guo, “Inhibition effect of inorganic and organic inhibitors on the corrosion of Mg–10Gd–3Y–0.5Zr alloy in an ethylene glycol solution at ambient and elevated temperatures,” Electrochimica Acta, vol. 56, pp. 10166-10178, 2011.
- H. Koch, M. P. H. Bronger, N. G. Thompson, Y P. Virmani, J.H. Payer, “Nace international,” Corrosion cost and preventive strategies in the United States. Avaliable: http://nace.org/uploadedFiles/Publications/ccsupp.pdf. [Access on 07 10 2013].
- Yohai, M. Vázquez, M.B. Valcarce, “Phosphate ions as corrosion inhibitors for reinforcement steel in chloride-rich environments,” Electrochimica Acta, vol 102, pp. 88-96, 2013.
- Samiento-Bustos, J.G. González Rodriguez, J. Uruchurtu, G. Dominguez-Patiño, V.M. Salinas-Bravo, “Effect of inorganic inhibitors on the corrosion behavior of 1018 carbon steel in the LiBr + ethylene glycol + H2O mixture,” Corrosion Science, vol. 50, issue 8, pp.2296-2303, 2008.
- Mohammad M. Fares, A.K. Maayta, Mohammad M. Al-Qudah, “Pectin as promising green corrosion inhibitor of aluminum in hydrochloric acid solution,” Corrosion Science, vol. 60, pp.112-117, 2012.
- Serpil Şafak, Berrin Duran, Aysel Yurt, Gülşen Türkoğlu, “Schiff bases as corrosion inhibitor for aluminium in HCl solution,” Corrosion Science, vol. 54, pp. 251-259, 2012.
- Elayyachy, A. El Idrissi, B. Hammouti, “New thio-compounds as corrosion inhibitor for steel in 1 M HCl,” Corrosion Science, vol 48, issue 9, pp. 2470-2479, 2005.
- S. Al-Otaibi, A.M. Al-Mayouf, M. Khan, A.A. Mousa, S.A. Al-Mazroa, H.Z. Alkhathlan, “Corrosion inhibitory action of some plant extracts on the corrosion of mild steel in acidic media,” Arabian Journal of Chemistry, In Press, Available online 8 February 2012.
- M Lagrenée, B Mernari, M Bouanis, M Traisnel, F Bentiss, “Study of the mechanism and inhibiting efficiency of 3,5-bis(4-methylthiophenyl)-4H-1,2,4-triazole on mild steel corrosion in acidic media,” Corrosion Science, vol. 44, issue 3, pp. 573-588, 2002.
- A. Ali, M.T. Saeed, S.U. Rahman, “The isoxazolidines: a new class of corrosion inhibitors of mild steel in acidic medium,” Corrosion Science, vol. 45, issue 2, pp. 253-266, 2003.
- Seifzadeh, H. Basharnavaz, A. Bezaatpour, “A Schiff base compound as effective corrosion inhibitor for magnesium in acidic media,” Materials Chemistry and Physics, vol 138, issue 2-3, pp. 794-802, 2013.
- Corrosion 2013 Program Preview, Materials Performance, Florida: Nace, 2013. Avaliable: http://mp.epubxp.com/i/105569. [Access on 07/10/2013].
- Jasna Halambek, Katarina Berković, Jasna Vorkapić-Furač, “Laurus nobilis L. oil as green corrosion inhibitor for aluminium and AA5754 aluminium alloy in 3% NaCl solution,” Materials Chemistry and Physics, vol. 137, Issue 3, pp. 788-795, 2013.
- A Aballe, M Bethencourt, F.J Botana, M Marcos, “CeCl 3 and LaCl 3 binary solutions as environment- friendly corrosion inhibitors of AA5083 Al– Mg alloy in NaCl solutions,” Journal of Alloys and Compounds, vol. 323–324, pp. 855- 858, 2001.
- Pandian Bothi Raja, Ahmad Kaleem Qureshi, Afidah Abdul Rahim, Hasnah Osman, Khalijah Awang, “Neolamarckia cadamba alkaloids as eco -friendly corrosion inhibitors for mild steel in 1 M HCl media,” Corrosion Science, vol. 69, pp. 292-301, 2013.
- A. Negm, N.G. Kandile, I.A. Aiad, M.A. Mohammad, “New eco -friendly cationic surfactants: Synthesis, characterization and applicability as corrosion inhibitors for carbon steel in 1 N HCl,” Colloids and Surfaces A: Physicochemical and Engineering Aspects, vol. 391, Issues 1–3, pp. 224-233, 2011.
- G Gunasekaran, L.R Chauhan, “Eco friendly inhibitor for corrosion inhibition of mild steel in phosphoric acid medium,” Electrochimica Acta, vol. 49, Issue 25, pp. 4387-4395, 2004.
- Sitashree Banerjee, Varsha Srivastava, M.M. Singh, “Chemically modified natural polysaccharide as green corrosion inhibitor for mild steel in acidic medium,” Corrosion Science, vol. 59, pp. 35-41, 2012.
- M. Abd El-Hafez, Waheed A. Badawy, “The use of cysteine, N-acetyl cysteine and methionine as environmentally friendly corrosion inhibitors for Cu-10Al-5Ni alloy in neutral chloride solutions,” Electrochimica Acta, In Press, Accepted Manuscript, Available online 1 July 2013.
- Khaled M. Ismail, “Evaluation of cysteine as environmentally friendly corrosion inhibitor for copper in neutral and acidic chloride solutions,” Electrochimica Acta, vol. 52, Issue 28, pp. 7811-7819, 2007.
- Junying Hu, Dezhi Zeng, Zhi Zhang, Taihe Shi, Guang-Ling Song, Xingpeng Guo, “2-Hydroxy-4-methoxy-acetophenone as an environment-friendly corrosion inhibitor for AZ91D magnesium alloy,” Corrosion Science, vol. 74, pp. 35-43, 2013.
- Gao, Q. Li, Y. Dai, F. Luo, H.X. Zhang, “High efficiency corrosion inhibitor 8-hydroxyquinoline and its synergistic effect with sodium dodecylbenzenesulphonate on AZ91D magnesium alloy,” Corrosion Science, vol. 52, Issue 5, pp. 1603-1609, 2010.
- Junying Hu, Daobing Huang, Guoan Zhang, Guang-Ling Song, Xingpeng Guo, “Research on the inhibition mechanism of tetraphenylporphyrin on AZ91D magnesium alloy,” Corrosion Science, vol. 63, pp. 367-378, 2012.